In a throwing environment:
1. Dri-fit shirt
2. Hair- Protein, water, lipids
C2H5NO2 C3H7NO2 (protein)
H2O (water)
C40H80NO8P (lipids)
3.Concrete
3CaOSO2, 2CaOSiO2, 3CaO Al2 O3, 4CaOAl2Fe2O3, CaSO4H2O
4. Sky/air
O2, N2
5.Discus- wood and steel
CH2O (wood/cellulose)
Fe3C (steel)
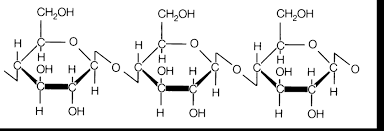 |
| Cellulose molecule |
6.Spandex- polyurethane-polyurea copolymer
C25H42N2O6
7.Grass
C6H10O5
8.Overhang-steel
Fe3C
9.Steel pole
Fe3C
 |
| Protein molecule |
10.Skin- protein, water, lipids
C2H5NO2 C3H7NO2 (protein)
H2O (water)
C40H80NO8P (lipids)
In a college students shopping trip:
1. Cardboard- cellulose
C6H10O5
2.Glass
SiO2
3.Linoleum
CaCO3 + C6H10O5
4.Fluorescent light
5.Plastic shelf- Diethylhexyl, polyethylene terephthalate
C6H4(COOC2H5)2
6.Cooling element- Chlorofluorocarbon
ClFC
7.Aluminum cooling element
AlC
8.Aluminum bottle caps
AlC
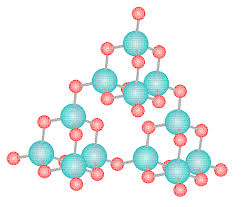 |
| SiO2 |
9.Beer
H2O, C2H5OH
10.Malt liquor
H2O, C2H5OH
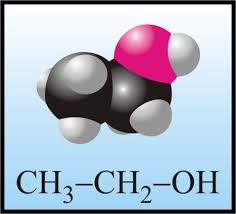 |
| Ethanol |